
The history…
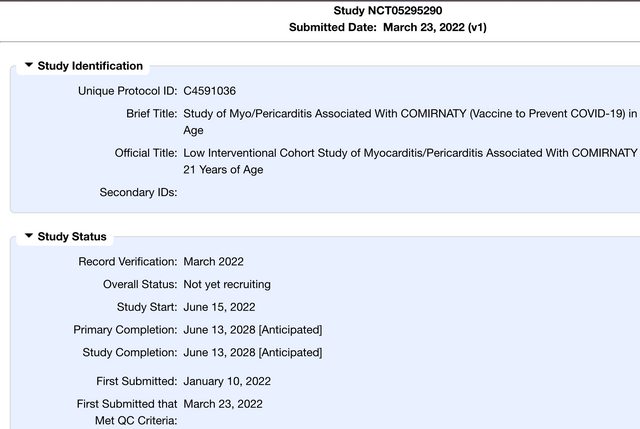
History of Changes for Study: NCT05295290
A Study to Learn About The COVID-19 (Study) Vaccine (Called COMIRNATY) in People That Are Less Than 21 Years Old.
https://classic.clinicaltrials.gov/ct2/history/NCT05295290
The study …
A Study to Learn About The COVID-19 (Study) Vaccine (Called COMIRNATY) in People That Are Less Than 21 Years Old.
ClinicalTrials.gov Identifier: NCT05295290
Sponsor: Pfizer
Collaborators: Carelon Research – National Health Lung Blood Institute
Information provided by (Responsible Party): Pfizer
Study Description
Brief Summary:
The purpose of this clinical trial is to learn about the safety and effects of the study vaccine (called COMIRNATY) for the potential prevention of COVID-19. This study is seeking participants who:Are age <21 years.
Have presentation to participating medical center with evaluation in Emergency Room and/or hospitalization.
Received either the 1st, 2nd, 3rd or booster dose(s) of COMIRNATY within 7 days of symptom onset.
Meet criteria of Centers for Disease Control and Prevention case definition of probable or confirmed myocarditis/pericarditis
Are capable of giving signed informed consent/assent (by parents/legal guardians of minors and/or patients), which includes compliance with the requirements and restrictions listed in the Informed Consent/Assent Document and in this protocol OR meets criteria for waiver of consent.
This study will examine the potential long-term effects associated with myocarditis/pericarditis following vaccination with COMIRNATY. The association of myocarditis/pericarditis in participants who received the study vaccine (COMIRNATY) compared with those associated with COVID-19 will also be examined. This will help us determine if COMIRNATY is safe and effective, and if there is a myocarditis/pericarditis association that should be noted. Participants will take part in this study for up to 5 years. During this time, they will receive complete cardiac imaging tests, and have follow up visits per guidance stated in the study protocol.
https://classic.clinicaltrials.gov/ct2/show/study/NCT05295290


